HEPA Box is the final air filtration device in the clean room, which needs to be periodically checked by DOP test to detect leaks and ensure standard filtration performance. The tutorial article “How to check HEPA Box performance by DOP test” helps maintain clean air quality according to GMP and ISO standards.
- 1. What is a HEPA Box and Why Should Its Performance Be Checked?
- 2. What is the DOP Test? Is It Mandatory?
- 3. DOP Testing Procedure for HEPA Boxes
- 4. How to Choose a HEPA Box Suitable for DOP Testing
- 5. Recommended DOP Testing Frequency by Industry
- 6. Benefits of Regular HEPA Box Testing
- 7. Common Questions about DOP Testing for HEPA Boxes
- 8. Contact Us - Professional DOP Testing Service, GMP Compliant
1. What is a HEPA Box and Why Should Its Performance Be Checked?
A HEPA Box is a terminal device in a cleanroom air filtration system, containing a HEPA filter (High Efficiency Particulate Air), which is responsible for removing up to 99.97% of airborne particles ≥0.3 microns before clean air enters the production area. This device is typically installed in ceilings or air ducts and plays a critical role in high cleanliness zones such as weighing rooms, filling areas, and packaging sections.
Why is performance verification necessary?
While HEPA filters offer high filtration efficiency, the overall effectiveness depends on several factors:
- Air-tightness between the filter and the housing
- Installation quality
- Physical condition of the filter (e.g., tears, folding, damage)
- Uniformity of clean air distribution
If the HEPA Box does not perform effectively, potential consequences include:
- Cross-contamination between clean and dirty zones
- Loss of cleanroom classification, resulting in product rejection or recall
- Inaccurate testing results, affecting batch quality
- Non-compliance during GMP, ISO, or HACCP audits

DOP Test - The Mandatory Performance Verification Method
To ensure HEPA Boxes function as intended, the DOP Test (Dispersed Oil Particulate Test) is widely applied to check for leakage and assess real-world performance of terminal filters. This test is mandated by several international standards, including:
- ISO 14644-3 (Annex B)
- EU-GMP Annex 1
- WHO-GMP
- NSF49 (for biological safety cabinets)
DOP testing is not merely a technical step—it is a critical validation criterion for contamination control in HVAC and cleanroom systems, especially for pharmaceutical, cosmetic, electronics, and food manufacturing environments.
See more: Latest price list of HEPA BOX used in medical clean rooms
2. What is the DOP Test? Is It Mandatory?
The DOP Test (Dispersed Oil Particulate Test) is a method used to verify the filtration efficiency and leakage integrity of HEPA filters in devices such as HEPA Boxes, FFUs, and biosafety cabinets. It is also referred to as a Leak Test, which detects whether particles bypass the filter media or leak through seals, gaskets, or installation points.
How does the DOP Test work?
- A certified aerosol (typically PAO - Poly Alpha Olefin) is introduced upstream of the filter.
- A photometer measures the particle concentration before and after the filter.
- If downstream levels exceed allowed limits (usually 0.01% or 0.03% of upstream concentration), the filter is deemed non-compliant.
Is the DOP Test mandatory?
Yes. It is a required procedure under several cleanroom standards:
|
Standard |
Requirement |
|
ISO 14644-3 |
Specifies leak test methods in Annex B |
|
EU-GMP Annex 1 (2022) |
Requires periodic filter testing in Grade A/B areas |
|
NSF49 |
Mandatory for biosafety cabinets using DOP or equivalent |
|
WHO-GMP, PIC/S |
Widely applied in pharmaceutical labs and manufacturing |
When should the DOP Test be performed?
|
Situation |
Purpose |
|
New HEPA Box installation |
Ensure system meets performance specifications from the outset |
|
Post-maintenance or filter replacement |
Verify air-tightness and filter efficiency after any intervention |
|
Periodic (6-12 months) |
Maintain cleanroom performance and support GMP compliance |
|
Pre-audit or requalification |
Provide technical validation documentation for inspections |
Failure to perform routine DOP testing not only increases contamination risk but can also lead to compliance failures in GMP, ISO audits, or customer assessments.
See more: HEPA Box Installation Best Practices
3. DOP Testing Procedure for HEPA Boxes
Conducting a DOP Test requires precise procedures, calibrated equipment, and trained personnel. Below is a standard step-by-step approach used in cleanroom environments:
1. Equipment Preparation
Ensure the following tools and PPE are ready before testing:
- Aerosol generator: Using DOP (dioctyl phthalate) or safer alternative PAO (polyalphaolefin)
- Photometer: Measures particle concentration upstream and downstream
- Access tools: Ladders, scaffolding, or integrated DOP test ports (if equipped)
- Personal protective equipment: Masks, safety goggles, gloves
2. DOP Test Procedure Steps
|
Step |
Description |
|
1 |
Inject aerosol into the airflow upstream of the HEPA filter |
|
2 |
Verify aerosol concentration meets standard levels for testing |
|
3 |
Use a photometer to scan the full surface of the filter downstream |
|
4 |
Mark and record any leak points detected (exceeding the threshold) |
|
5 |
Compare with standards: Pass if leakage is ≤0.01% (GMP) or ≤0.03% (ISO) of upstream level |
Note: The scan must be performed slowly, continuously, and cover the entire surface—including filter edges, frame joints, and any potential leak zones.
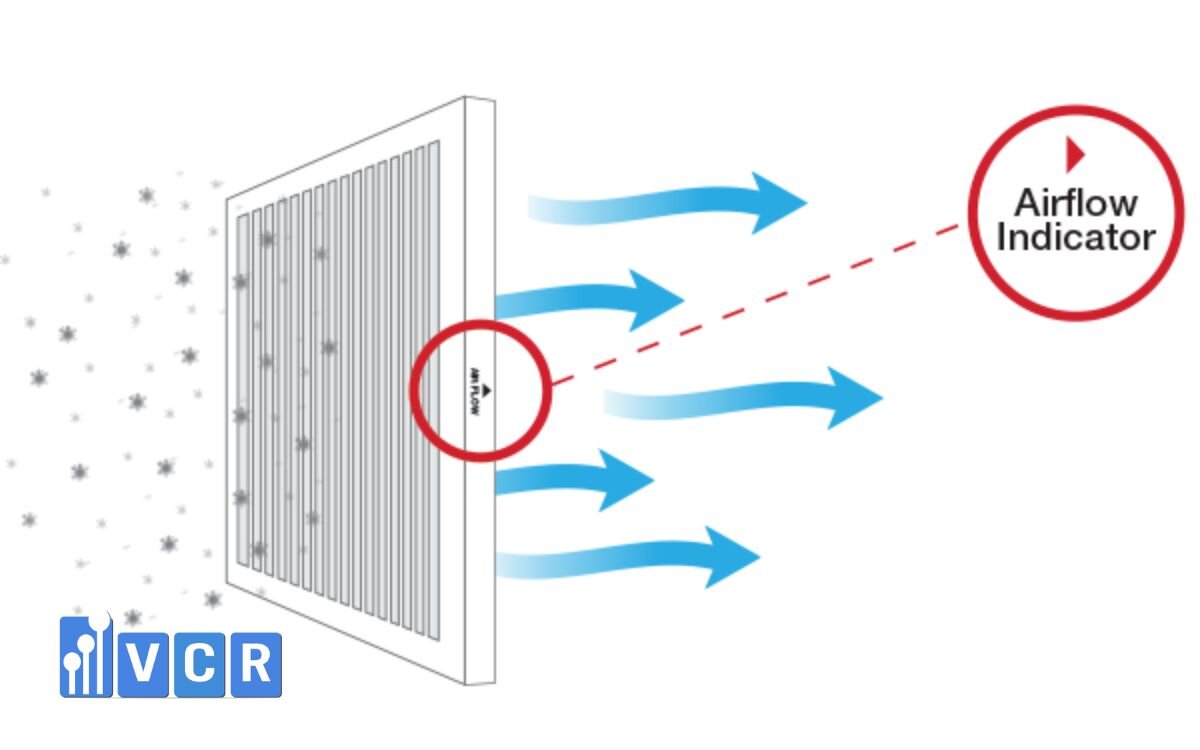
3. Safety Considerations & Common Mistakes
To ensure reliable DOP Test results, avoid these frequent issues:
- Uncalibrated photometer → Produces inaccurate readings
- Uneven aerosol injection or insufficient concentration → Misses small leaks
- Partial surface scanning → Critical leak zones may be overlooked
- Improper PPE or poor ventilation → Aerosol inhalation risk (especially with DOP)
Adhering to a validated DOP Test protocol not only verifies the HEPA Box’s performance, but also safeguards production integrity and strengthens regulatory audit readiness.
See more: Verifying HEPA Box Performance for Cleanroom Suitability
4. How to Choose a HEPA Box Suitable for DOP Testing
Not all HEPA Boxes are optimized for performance testing using the DOP method. Choosing the right model from the beginning can reduce testing time, improve accuracy, and lower long-term validation costs.
Below are key criteria to consider if you plan to perform regular DOP tests:
1. Prioritize models with an integrated DOP test port
A DOP port is a connection point installed directly on the HEPA Box that allows aerosol injection into the upstream side and particle measurement without dismantling the duct system.
It enables easy and fast routine testing, especially in hard-to-access locations.
The port should follow standard design with an airtight cap when not in use.
Some HEPA Boxes from VCR already include built-in DOP ports, simplifying the testing process and meeting GMP inspection requirements.
2. Use gel-seal HEPA filters to prevent leakage
HEPA filters should have a gel-seal instead of a standard gasket.
Gel-seal fills all gaps between the filter and the housing, minimizing the risk of air leakage at contact points—a common issue during DOP tests.
This is especially important in Grade A/B cleanroom zones in pharmaceutical production.

3. Airtight box housing with easy technician access
The HEPA Box frame should be made from stainless steel or powder-coated steel with welded seams to prevent air leaks.
The design must allow technicians to easily access the entire downstream surface of the filter for scanning with a photometer.
If a diffuser is included, it should be removable for thorough testing.
4. Product suggestion from VCR: HEPA Box with integrated DOP port
Recommended model: VCR-HB-DOP Series
Key features:
- Integrated standard DOP test port (⌀12mm)
- Gel-seal HEPA filter with ≥99.99% efficiency
- Airtight stainless steel 304 housing with removable diffuser
- Available with technical drawings and CO/CQ certificates to meet GMP validation requirements
5. Recommended DOP Testing Frequency by Industry
The frequency of DOP testing for HEPA Boxes varies by industry. It depends on environmental control levels, applicable standards, and product sensitivity. Companies should establish appropriate testing schedules based on the following:
|
Industry |
Recommended Frequency |
Notes |
|
Pharmaceuticals (GMP) |
Every 6-12 months |
Mandatory per EU-GMP/WHO-GMP, especially for Grade A/B areas |
|
Cosmetics (ISO 22716) |
Annually |
Linked to supplier audits or periodic requalification |
|
Food (HACCP) |
Annually |
Applies if cleanrooms or controlled zones are in use |
|
Electronics (ISO 14644) |
Depends on cleanroom class |
ISO 5-6 should test every 6-9 months to prevent ultra-fine particle contamination |
Note: In addition to routine schedules, HEPA Boxes must also be retested after filter changes, repairs, or before major audits.
See more: HEPA Box Enclosures for Gel-Seal Filters in Pharmaceutical Cleanrooms
6. Benefits of Regular HEPA Box Testing
HEPA Box testing should not be viewed as a regulatory burden but as an investment in cleanroom and product protection. Key benefits include:
1. Ensures product quality
- Minimizes contamination from dust, microbes, and foreign particles
- Reduces the risk of batch failure due to environmental impurities, especially for pharmaceutical, cosmetic, or precision electronics products
2. Ensures compliance and audit readiness
- Complete test reports support successful GMP, ISO, and HACCP audits
- Strengthens supplier ratings in global manufacturing chains

3. Optimizes cost and extends system lifespan
- Early detection of damage enables timely maintenance, saving energy costs
- Helps HVAC systems operate efficiently, reducing fan and filter wear
4. Prevents unexpected cleanroom classification loss
- Filter leaks can cause entire cleanroom zones to fall out of spec, disrupting production and damaging company credibility
7. Common Questions about DOP Testing for HEPA Boxes
Is it okay to skip DOP testing?
No. Without testing, leaks in the HEPA filter or housing may go unnoticed, leading to:
- Loss of cleanroom classification
- Cross-contamination or inaccurate testing results
- Violations of GMP, ISO standards, increasing audit failure risk
Can we conduct DOP testing in-house?
Yes, if your facility has the proper equipment and trained technicians. However, in most cases, it is advisable to:
- Hire a certified testing provider with compliant equipment and validated reporting
- Ensure objectivity, transparency, and regulatory compliance
What is the difference between DOP and PAO?
- DOP (Dioctyl Phthalate): Traditional substance used in leak testing, but potentially harmful if inhaled long-term
- PAO (Poly Alpha Olefin): Safer, non-toxic alternative widely recommended for modern facilities
Today, most testing companies use PAO to ensure occupational safety.
Should DOP testing be repeated after filter replacement?
Yes, it is mandatory. Each time a HEPA filter is changed or the system is serviced, DOP testing must be performed to:
- Confirm no installation-related leaks
- Verify continued filter performance
- Ensure the system maintains the original cleanroom specification
8. Contact Us - Professional DOP Testing Service, GMP Compliant
Need to validate your HEPA Boxes according to GMP or ISO standards?
VCR offers professional DOP Testing services with the following commitments:
- Testing equipment meets ISO 14644-3 standards
- Uses PAO aerosol - safe and non-toxic
- Experienced technicians with GMP-compliant procedures
- Clear, detailed reports - suitable for audits and requalification
Hotline: 090.123.9008
Email: [email protected]
Website: https://hepabox.vn/
Diep VCR