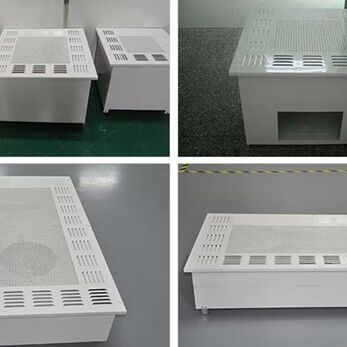
The HEPA Box is an essential air filtration device in cleanrooms, helping to maintain air cleanliness that meets GMP standards. But do low-cost HEPA Box models truly ensure the required filtration efficiency and airtightness? This article will help you make a comprehensive evaluation before making your choice.
This article from VCR will help you answer the question: “HEPA Box at a low price: can it meet GMP standards?” - from the concept of HEPA Box, the requirements of GMP, to assessing whether a low-cost device is suitable for GMP‐compliant clean-room production environments.
1. What is a HEPA Box?
A HEPA Box is a box-type air-filtration device that integrates a HEPA (High Efficiency Particulate Air) filter and is typically installed in the ceiling of cleanrooms. With a compact but highly effective design, this device plays a key role in maintaining the cleanliness of the air according to strict standards.
Main functions of the HEPA Box
-
Filters fine particulates and microorganisms down to 0.3 micron in size with efficiency up to 99.97% (for HEPA H13 or higher).
-
Supplies unidirectional clean air (laminar flow), helping to prevent cross-contamination between production zones.
-
Stabilises pressure and air volume flow, supporting maintenance of clean-room classification according to GMP or ISO 14644.
Common applications of the HEPA Box
-
Pharmaceuticals: installation in sterile production areas, injection drug manufacturing areas, Class A/B/C zones.
-
Food: used in packaging lines, filling rooms, where clean air and biosafety are required.
-
Cosmetics: controlling the environment in the production of premium skincare products, serums.
-
Electronics / Semiconductors: SMT cleanrooms, PCB assembly areas, zones where dust particles affect micro-circuits.
As the “final filter” in an HVAC system, a HEPA Box is indispensable in assuring the production environment quality meets GMP standards.

Read more: Structure and application of HEPA Box for ISO 5 clean room
2. What do GMP standards require of air filtration systems?
In GMP-compliant production facilities-for pharmaceuticals, cosmetics, or nutritional supplements-air-filtration systems play a pivotal role in maintaining a clean environment, preventing cross-contamination and ensuring product quality. Below are the core technical requirements that devices like the HEPA Box must satisfy:
Air cleanliness classification (ISO Class / Federal Standard)
-
GMP requires the production environment to achieve cleanliness levels corresponding to ISO 5 (Class 100), ISO 7 (Class 10 000) or ISO 8 (Class 100 000), depending on the zone.
-
The HEPA filter integrated in the HEPA Box must be capable of removing ≥ 99.97% of particles ≥ 0.3 micron in order to keep air quality within control limits.
-
In sterile production areas, air cleanliness is frequently evaluated via particle count (counting suspended particulates).
Air flow rate and velocity
-
Ensure sufficient clean air volume to continuously replace contaminated air.
-
Air velocity through the surface of the HEPA filter generally ranges from 0.45 to 0.6 m/s, to keep the airflow stable and reduce back-flow contamination.
-
If airflow is uneven or too low, dead zones may form where microorganisms or fine dust accumulate.
Pressure differential between zones
-
Pressure differential ensures air moves from cleaner zones to less clean zones, avoiding reverse contamination.
-
GMP recommends maintaining a minimum differential of 10-15 Pa between cleanliness levels.
-
The HEPA Box must be integrated with a differential pressure gauge to monitor the pressure in a timely manner.
Ability to perform periodic inspection and maintenance
-
Air-filtration equipment must be designed for easy disassembly, filter replacement, internal cleaning.
-
A differential pressure gauge or alarm is needed to indicate filter clogging or performance degradation.
-
The casing material must be corrosion-resistant, easy to clean, and not generate secondary particulates.
Required documentation & verification
-
According to cleanroom validation procedures, air-filtration equipment must include documentation such as:
-
DOP test: checks HEPA filter efficiency using aerosol of oil particles
-
Airflow test: measures the direction and velocity of airflow
-
Particle count: measures the density of suspended particles in the air
-
-
These documents are required in HVAC system validation and GMP audits.

Read more: HEPA Box Installation Best Practices
3. What typical differences appear in low-cost HEPA Boxes?
A HEPA Box is an important component in a cleanroom HVAC system, directly affecting air cleanliness and the ability to obtain GMP certification. However, not all units are manufactured to the same standard. Low-cost HEPA Boxes often exhibit several noteworthy differences:
Regarding construction materials
-
Many low-cost HEPA Boxes use thin stainless steel (often below 0.8 mm), prone to deformation during installation or long-term operation.
-
Some devices do not use SUS 304 stainless steel (GMP standard) but instead use SUS 201 steel or even powder-coated steel, which risks corrosion, rusting, and harder cleaning.
-
Note: GMP requires durable materials, non-particle-emitting and easily washable, while poor-grade stainless steel may fail these demands.
Regarding the HEPA filter media
-
To reduce cost, some manufacturers use filter grades H11 or H12 - which do not meet EN 1822 standard for the most stringent cleanrooms. These filters may only achieve ~95-99.5% efficiency, whereas H13 or higher filters meet removal of ≥ 99.97% of particles ≥ 0.3 µm.
-
This directly affects the ability to control microorganisms and particulates - vital criteria for GMP audits.
Regarding performance testing and certifications
-
Low-cost HEPA Boxes often come without DOP test reports (Dispersed Oil Particulate), meaning they cannot demonstrate actual filtration performance.
-
Many such devices lack differential pressure gauges and do not support monitoring filter clogging or performance decline.
-
Lack of verification data means the plant will face difficulty in risk assessment and maintaining the controlled state.
Regarding air-tightness during installation
-
Another weakness is imprecise manufacturing/assembly: poor sealing, low-quality or missing gaskets, leading to air leakage at seams.
-
This is a key reason why a “Class C” zone might fall to “Class D” simply due to a leaky HEPA Box.
-
A cleanroom could fail its classification not because of the HVAC system, but due to leakage from the ceiling HEPA Box.
Read more: Unveiling the HEPA Box: Guardian of Clean Air in Critical Environments
4. Should you use a low-cost HEPA Box in a GMP-compliant plant?
Using a low-cost HEPA Box may help reduce initial investment. However, in an environment that must comply with Good Manufacturing Practice (GMP)-where any contamination risk directly affects product quality-the decision needs careful consideration.
a. Cases where you should NOT use a low-cost HEPA Box
-
A plant that has already achieved GMP certification: In this case any equipment or design change must comply with strict requirements. Using a sub-standard HEPA Box could result in:
-
Failing an audit from the Ministry of Health or international partners
-
Incurring retrospective remediation costs if the equipment causes deviations in environmental parameters
-
-
Areas requiring Class A/B/C cleanliness: These are high cleanliness levels, typically in:
-
Production of injectable drugs, eye-drop pharmaceuticals, sterile products
-
Patching/packaging rooms for microbe-sensitive products
-
-
Without budget for periodic inspection/calibration: Some low-cost HEPA Boxes may work for a short time, but to ensure safety, you must regularly:
-
Check filtration performance
-
Calibrate differential pressure gauges and airflow
-
Schedule filter replacement on time
-
-
If the enterprise lacks budget for these activities, choosing a low-quality HEPA Box is a major risk.
b. Cases where you MAY consider using a low-cost HEPA Box
-
Support zones with minimal microbial control requirements: e.g.,
-
Technical corridors
-
Low-level changing rooms
-
Storage rooms for unopened materials
These may only require ISO Class 8 or Class D. In such cases a reasonably-priced HEPA Box may suffice if still meets the dust-filtration design.
-
-
Pilot projects or layout demo phases: During pilot trials of production lines or cleanrooms, or when evaluating layout options, you may use low-cost equipment to reduce investment. But you must note:
-
Use only for a short time
-
Do not use in main operation before full evaluation
-
-
If the device still comes with proof of performance: Some domestic low-cost HEPA Boxes may still provide:
-
Clear CO/CQ
-
DOP test results, technical drawings, airflow report
-
Transparent warranty and after-sales policy
If these are present and the equipment passes on-site testing (Site Acceptance Test – SAT), then you may consider using it in zones with low or moderate control requirements.
-
Read more: Verifying HEPA Box Performance for Cleanroom Suitability
5. How to evaluate whether a HEPA Box meets GMP or not?
Choosing a “good value” HEPA Box that still meets GMP standards is absolutely feasible - if you know how to evaluate it correctly. Below are 3 mandatory technical factors you must check:
Verification documents: CO/CQ and HEPA filter testing
- CO (Certificate of Origin) and CQ (Certificate of Quality) are the first documents proving product origin and quality.
- A GMP-compliant HEPA Box must come with filtration performance test documents such as:
-
-
-
DOP Test (Dispersed Oil Particulate): checks filtration efficiency for 0.3 µm particles with ≥ 99.97% removal.
-
MPPS Test (Most Penetrating Particle Size): measures precise filter grade per EN 1822 (H13, H14, etc.).
-
-
- If the supplier cannot provide these documents, the device almost certainly does not meet GMP standards.
Check the detailed specification
- When reviewing information about the HEPA Box, you should compare the following parameters against GMP requirements:
-
-
-
Filter grade: minimum H13 (EN 1822), preferably H14
-
Air velocity: 0.35-0.45 m/s (depending on zone)
-
Casing material: stainless steel 304 or powder-coated steel, thickness ≥ 1.0 mm
-
Filter panel size: suitable for cleanroom ceiling, easy to replace
-
Differential pressure gauge: present to monitor filter clogging
-
-
- Many low-cost models only use H11-H12 filters or lack filter grade information - not acceptable for sterile production areas.
Prioritise suppliers with GMP experience
- A GMP-compliant HEPA Box is not just about the product, but also about the supplier’s capability to consult and support implementation. You should prioritise suppliers who:
-
-
Have real GMP cleanroom projects implemented
-
Have engineers who understand ISO 14644 and WHO GMP
-
Support on-site DOP testing, periodic calibration
-
-
- A “cheap” supplier without understanding GMP requirements could cost you dearly during an audit.
Quick checklist to evaluate if a HEPA Box is GMP-compliant:
-
Does the HEPA filter meet H13 or H14, and is that clearly stated?
-
Is a differential pressure gauge provided for monitoring filter clogging?
-
Is there a filtration performance test report: DOP/MPPS?
-
Are technical drawings and specifications clearly provided?
-
Has the supplier carried out GMP-project implementations?

Read more: Supplier of GMP‑Standard Cleanroom HEPA Box Filters
FAQ – Frequently asked questions
Does a low-cost HEPA Box always mean low quality?
Not necessarily. Low price does not always equate to low quality if the device meets fundamental technical standards. Some domestic suppliers reduce costs through:
- Large scale in-house production, local machining
- Minimised marketing expenses, direct sales channels
- Using locally-sourced materials instead of imports
However, the essential condition is that the HEPA Box still must: - Use filters conforming to EN 1822 (H13 or higher)
- Have filtration performance results such as DOP, MPPS
- Ensure airtight installation, no leakage of contaminated air
If any of these are missing, even a good price is still not suitable for a GMP environment.
Can I use a low‐cost HEPA Box in a changing room?
Yes, if the zone doesn’t require strict microbial control. Specifically:
- A Class D changing area or an unclassified area may use mid-level HEPA Box.
- A zone that only requires dust filtration and not microbial testing.
However, you should still ensure: - A differential pressure gauge to monitor filter clogging
- Proper installation sealing, so it doesn’t affect clean-air airflow to main production areas
If the changing room is Class C or B, you should not use equipment lacking filtration performance certification.
Is a device without a DOP test accepted in a GMP audit?
No. In a GMP cleanroom system, air-filtration equipment (including HEPA Boxes) must be tested for filtration performance with methods such as:
- DOP Test: checks leakage and filtration effectiveness on-site
- MPPS Test: performed at the factory according to EN 1822
A device without these certification tests typically: - Cannot demonstrate ability to filter microorganisms
- Will not satisfy audit requirements
- Carries a risk of the enterprise losing certification or having to remediate after the fact
Therefore, despite a low price, a HEPA Box without verified performance is not suitable for microbial-controlled zones such as sterile production or pharmaceutical packaging.
Need a HEPA Box at a good price but compliant with GMP?
Don’t let a low-cost device cause you to lose your cleanroom standard! Choose a HEPA Box that is truly compliant, with full testing from a supplier who understands GMP (such as VCR).
-
Industry-specific consultation: Pharmaceuticals – Cosmetics – Food – Electronics
-
Provides full CO, CQ, DOP testing
-
Optimises cost while ensuring microbial control
Contact:
Hotline: 090.123.9008
Email: [email protected]
Website: https://hepabox.vn/
Dat VCR