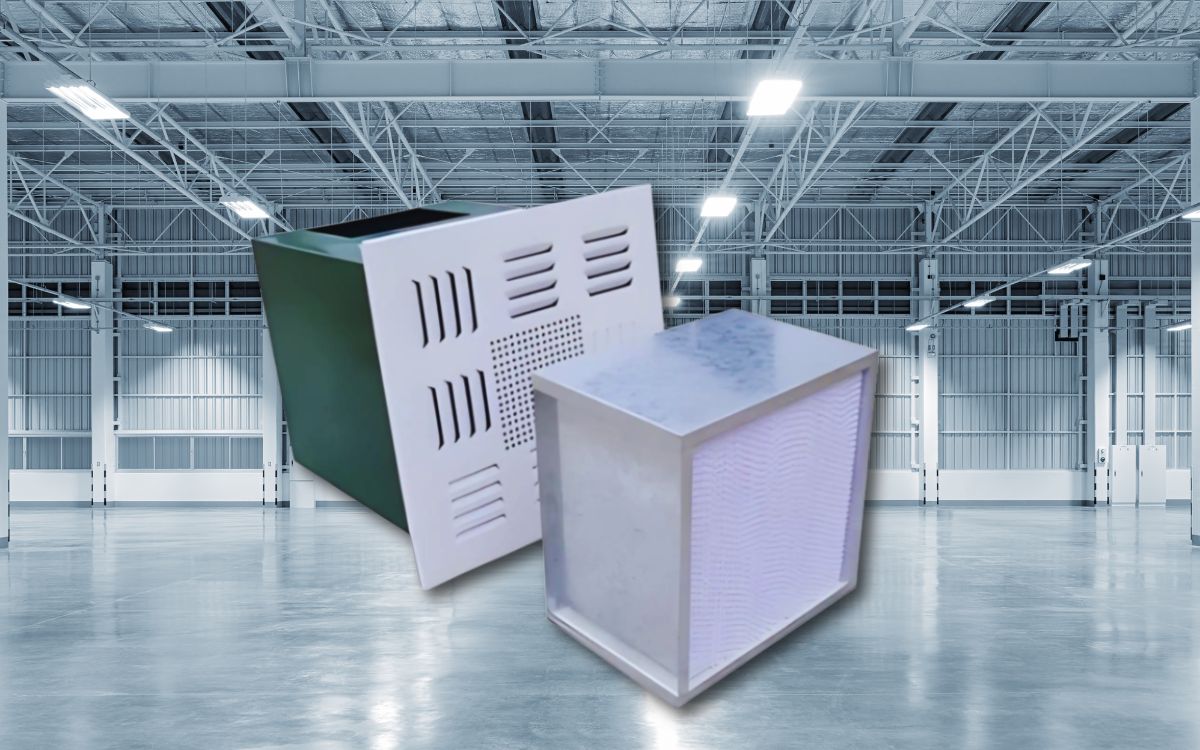
HEPA Box is the terminal of the clean room air filtration system, helping to distribute clean air flow according to ISO and GMP standards. However, if installed incorrectly such as open frame, wrong air direction or not checking the tightness, the filtration efficiency will decrease
- 1. What is a HEPA Box? Its Role in Cleanroom Air Filtration Systems
- 2. Common Installation Errors of HEPA Boxes and How to Fix Them
- 3. HEPA Box Installation Checklist
- 4. Suggested Applications of HEPA Box by Industry
- 5. Frequently Asked Questions About HEPA Box Installation
- 6. Need Help Choosing or Installing a HEPA Box?
1. What is a HEPA Box? Its Role in Cleanroom Air Filtration Systems
A HEPA Box is the terminal device of a cleanroom air filtration system, designed to house a HEPA filter (High Efficiency Particulate Air). It is typically installed at the final air supply points before clean air is distributed into the controlled area.
Basic Components of a HEPA Box:
- Casing made of stainless steel or powder-coated steel
- HEPA filter mounting frame
- Air duct connection (round or square)
- Air diffuser (optional)
- Some models include a pressure gauge or air damper

Applications of HEPA Box in Different Cleanroom Classes
HEPA Boxes play a crucial role in:
- Ensuring cleanliness levels that meet ISO 5-8, GMP, or equivalent standards
- Preventing fine dust, microorganisms, and unwanted gases from entering the workspace
- Distributing clean air evenly and maintaining laminar airflow
Depending on the required cleanliness level and airflow rate, HEPA Boxes are used as follows:
|
Cleanroom Class |
Typical Application |
Filter Type |
|
ISO 5 |
Sterile filling room in pharmaceutical plants |
ULPA or HEPA H14 |
|
ISO 6 |
Weighing room, electronic component testing area |
HEPA H14 |
|
ISO 7-8 |
Packing or final inspection area |
HEPA H13 or H14 |
2. Common Installation Errors of HEPA Boxes and How to Fix Them
Installing a HEPA Box may seem simple, but in practice, it requires precision to ensure filtration efficiency and compliance with cleanroom standards. Below are five common mistakes engineers or contractors often make:
Error 1: Incorrect Airflow Orientation
Consequences:
- Causes airflow turbulence
- Disrupts laminar flow in the production zone
- Makes it difficult to achieve the required ISO cleanliness level
Common Causes:
- Installed too close to doors or heat-generating equipment
- HVAC layout not followed during installation
Solution:
- Identify correct airflow direction: vertical (from ceiling) or horizontal (from wall)
- Follow the approved HVAC layout drawing
- Use CFD simulation during design if possible
Error 2: Failure to Check for Leakage
Consequences:
- Unfiltered air may leak through gaps
- Contaminates the workspace with fine dust or microbes
- Leads to loss of air quality control
Solution:
- Perform leakage testing using PAO/DOP method after installation
- Seal all joints with HVAC-grade silicone
- Record test results for reference during operation

Error 3: Using the Wrong Filter Type (HEPA vs. ULPA)
Typical Cases:
- Pharmaceutical plants require H14 filters but H13 filters are installed
- Electronics cleanrooms use ULPA filters without verifying efficiency
Consequences:
- Fails to meet GMP or ISO 14644 requirements
- Risk of cross-contamination or component failure
Filter Selection Guide:
|
Filter Type |
Efficiency |
Suitable Application |
|
HEPA H13 |
≥99.95% |
Food, cosmetics, basic cleanrooms |
|
HEPA H14 |
≥99.995% |
GMP pharmaceutical, ISO 6 electronics |
|
ULPA |
≥99.9995% |
ISO 5 or higher, sterile environments |
Error 4: Not Monitoring Differential Pressure
Consequences:
- Reduced filtration performance over time
- Increased system resistance and energy consumption
- Shortened equipment lifespan
Solution:
- Install a differential pressure gauge (analog or digital) on each HEPA Box
- Check regularly; replace the filter if pressure exceeds the threshold
- Integrate with the BMS system for automatic alerts if available
Error 5: Lack of Cleaning or Filter Replacement Schedule
Consequences:
- Dust buildup reduces airflow
- Microbial growth can occur, affecting product quality and operator safety
- Higher energy consumption
Recommended Maintenance Schedule:
|
Industry |
Filter Replacement Frequency |
PAO/DOP Test |
|
Pharmaceutical (GMP) |
Every 6-12 months |
Mandatory |
|
Electronics |
Every 12 months |
Recommended |
|
Food & Cosmetics |
Every 12-18 months |
Annual check |
Xem thêm: HEPA Box in Microbiology: Ensuring ISO 5 Environment
3. HEPA Box Installation Checklist
Installing a HEPA Box is more than simply attaching it to the ceiling or wall. To ensure the cleanroom operates within standards, engineers must follow a precise inspection process. The checklist below helps verify key compliance items:
|
Inspection Item |
Standard Requirement |
Inspection Frequency |
|
Leakage Test (PAO/DOP) |
No detectable leakage |
Upon installation, every 6 months |
|
Filter Differential Pressure |
Within manufacturer specifications |
Monthly |
|
Installation Position |
Correct airflow direction (vertical/horizontal) |
During installation and adjustment |
|
Filter Type |
Correct grade: HEPA H14 / ULPA (if required) |
Every replacement |
Important Notes:
- Maintain complete inspection records for GMP, ISO, or HACCP audits
- Use differential pressure gauges to detect filter degradation early
- Assign a responsible technician for all air filtration validation tasks
4. Suggested Applications of HEPA Box by Industry
HEPA Boxes are not just general air filtration devices for cleanrooms—they must be selected and installed according to the specific technical requirements of each industry. Below are practical application guidelines for three key sectors:
Pharmaceutical Industry - Complying with EU-GMP Standards
In pharmaceutical production, particularly in weighing, dispensing, and compounding areas, strict control of cleanliness and cross-contamination is required.
HEPA Box Applications:
- Use HEPA H14 filters with ≥99.995% efficiency
- Install in weighing rooms, airlocks, and compounding zones
- Combine with differential pressure gauges and routine PAO testing

Electronics Industry - Static and Fine Particle Control
Electronics manufacturing, especially for semiconductors and SMT components, requires ultra-clean environments (ISO 5-6) and electrostatic control.
HEPA Box Applications:
- Use both FFU and HEPA Boxes in areas requiring strict particulate control
- Prioritize HEPA H14 or ULPA filters for fine electronics production lines
- Ensure stable unidirectional airflow layout, avoiding interference from machines
Food Industry - Focus on Moisture and Hygiene Control
In food processing plants, HEPA Boxes are typically installed in areas with high risk of dust, odor, or microbial growth such as packaging, filling, and storage zones.
HEPA Box Applications:
- Select models with easy-to-replace filters, suitable for regular cleaning
- Choose powder-coated steel or stainless steel (304) casings for corrosion resistance
- Integrate with humidity control systems to prevent mold buildup inside filters
Xem thêm: How to test HEPA Box performance with DOP test
5. Frequently Asked Questions About HEPA Box Installation
1. Is there a difference between a HEPA Box and an FFU?
Yes. A HEPA Box is a terminal unit that holds a HEPA filter but requires an external air supply. An FFU (Fan Filter Unit) integrates both a fan and a HEPA filter and can operate independently. HEPA Boxes are commonly used in centralized HVAC systems, while FFUs are better suited for small-scale or retrofit cleanrooms.
2. How do I check if a HEPA Box is leaking?
The standard method is PAO/DOP leak testing. Technicians use aerosol generators and scanning devices to detect microscopic leaks that are not visible to the naked eye. This test is mandatory in pharmaceutical facilities and should be conducted every 6 months.
3. Can a HEPA Box be used in an ISO 5 cleanroom?
Yes, but it must use HEPA H14 or ULPA filters, be properly installed with correct airflow orientation, and maintain unidirectional laminar flow. In practice, ISO 5 rooms often use FFUs or dedicated AHU systems for more stable airflow control.
4. How often should HEPA filters be replaced?
Filter replacement frequency depends on industry and usage:
- Every 6-12 months for pharmaceutical and electronics sectors
- Every 12-18 months for food and cosmetics sectors
Regular monitoring of differential pressure and filtration performance helps determine the right replacement time.
5. Can a HEPA Box be reused?
The HEPA Box casing can be reused if not damaged. However, the HEPA filter is a consumable and must be replaced according to schedule. Filters should not be washed or reused under any circumstances.
6. Need Help Choosing or Installing a HEPA Box?
With over 10 years of experience in cleanroom solutions, VCR provides not only internationally certified equipment but also full support in design, validation, and installation tailored to each industry.
- Technicians with deep knowledge of GMP, ISO, HACCP
- Consulting to select the right HEPA Box based on actual layout
- Support for PAO/DOP testing, pressure monitoring, and periodic maintenance
Contact VCR’s cleanroom experts today:
Hotline: 090.123.9008
Email: [email protected]
Website: https://hepabox.vn/
Diep VCR